Large differences in transcriptional networks of normal and tumor colon cells
Cordero D, Solé X, Guinó E, Sanz-Pamplona R, Berenguer A, Moreno V.
Unit of Biomarkers and Susceptibility, Cancer Prevention and Control Program. ICO-IDIBELL.
Introduction
Transcriptional regulatory programs have an essential role in cancer. Although specific alterations are well described, studies at the whole-genome level are required to obtain more information about the transcriptional programs involved in the tumor development.
Objectives
Our aim is to characterize the differences between transcriptional programs of normal and tumor colon cells, through a reverse engineering reconstruction of gene regulatory networks.
Methods
Genome-wide gene expression profiles for 196 colon cancer cases (98 tumors and 98 paired normal tissues) were obtained using microarrays. Regulatory networks for both normal and tumor samples were built using the ARACNe algorithm. Kernel bandwidth and mutual information null distribution were previously estimated for the dataset. For each cell type, 1000 bootstrap replicates were performed and summarized to obtain accurate consensus networks. Visualizations and topological network analyses were performed with the Cytoscape platform. Overrepresentation of Gene Ontology categories in these biological networks was carried out with the Cytoscape plugin BINGO. For additional analyses and data processing, the R statistical environment was used.
Results
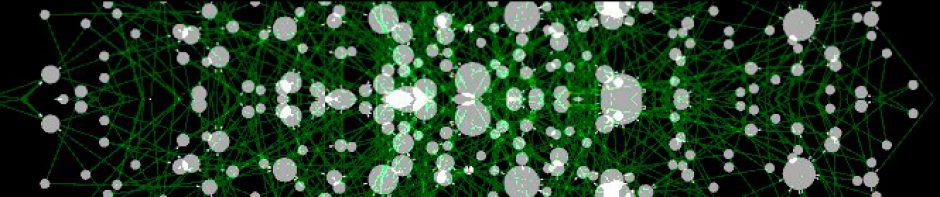
A large loss of transcriptional interactions in the tumor regulatory network was found (Fig. 1). The tumor regulatory network contained 37% fewer transcription factors than the regulatory network of normal cells (755 vs. 1185), as well as 56% fewer target genes (2384 vs. 5471). Furthermore, the number of direct transcriptional interactions is reduced an 80% (11940 in the tumor network vs. 61235 in the regulatory network of normal cells). This fact suggests that the loss of interactions may not be only due to potential alterations at the DNA level, but also because of failures in the transcriptional machinery of the tumor. Notably, there are also specific transcription factors that hugely increase their number of target interactions in the tumor network, as displayed for GREM1 in Fig. 2. Functional analysis of tumor regulatory network displayed classical cancer-related biological processes significantly enriched such as immune response (adj. pval=1.13E-5) or developmental process (adj. pval=0.0002), among others.
Conclusions
Inference of gene regulatory networks at the whole-genome level has allowed us to detect a generalized loss of transcriptional activity in colon tumors, which had not been described before. This finding will allow a better comprehension of the transcriptional regulatory programs altered in colon cancer.