REFERENCE
Sanz-Pamplona R, Berenguer A, Cordero D, Molleví DG, Crous-Bou M, Sole X, Paré-Brunet L, Guino E, Salazar R, Santos C, de Oca J, Sanjuan X, Rodriguez-Moranta F, Moreno V.
Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer.
Mol Cancer. 2014 Mar 5;13(1):46. PubMed PMID: 24597571.
ABSTRAC
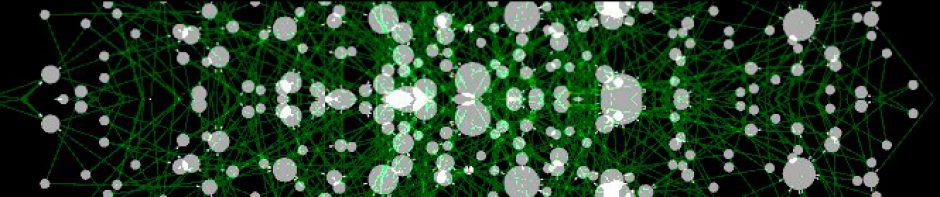
Background: A colorectal tumor is not an isolated entity growing in a restricted location of the body. The patient’s gut environment constitutes the framework where the tumor evolves and this relationship promotes and includes a complex and tight correlation of the tumor with inflammation, blood vessels formation, nutrition and gut microbiome composition. The way in which the tumor influences their environment could both promote an anti-tumor or a pro-tumor response. Methods: A set of 98 paired adjacent-normal and tumor tissues from CRC patients and 50 colon mucosa from healthy donors (246 samples in total) were included in this work. RNA extracted from each sample was hybridized in Affymetrix chips Human Genome U219. Functional relationships between genes were inferred by means of systems biology using both transcriptional regulation networks (ARACNe algorithm) and protein-protein interaction networks (BIANA software). Results: Here we report a transcriptomic analysis revealing a number of genes activated in adjacent mucosa from CRC patients, not activated in mucosa from healthy donors. A functional analysis of these genes suggested an active reaction of the adjacent mucosa related to the presence of the tumor. Transcriptional and protein-interaction networks were used to further elucidate this response of normal gut in front of the tumor, revealing a crosstalk between proteins secreted by the tumor and receptors activated in the normal colon tissue; and vice versa. Remarkably, Slit family of proteins activated ROBO receptors in tumor whereas tumor-secreted proteins transduced a cellular signal finally activating AP-1 in normal tissue. Conclusions: The systems-level approach provides new insights into the micro-ecology of colorectal tumorogenesis. Disrupting this intricate molecular network of cell-cell communication and pro-inflammatory microenvironment could be a therapeutic target in CRC patients.